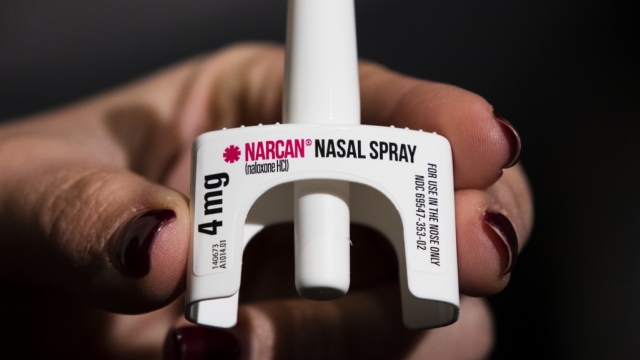
A lifesaving medication used in cases of opioid overdoses could be hitting store shelves by as soon as next week.
The U.S. Food and Drug Administration approved Naloxone earlier this year to start being sold directly to consumers. The nasal spray, which is commonly known by its brand name Narcan, will become the first opioid treatment drug to be sold over-the-counter without the need for a prescription. It can reverse overdoses of opioids, including street drugs such as heroin and fentanyl and prescription versions including oxycodone.
SEE MORE: Scripps News finds red flags in review of child fentanyl overdoses
Emergent, the company that produces the overdose antidote, says it plans to sell the nasal spray product for less than $50 for a box of two doses. The company said its goal is to match the price that government agencies, nonprofit organizations and first responders pay on average.
"A goal for the out-of-pocket retailer price is to be consistent with our public interest pricing for one carton of two 4 mg doses, although retail price is set by individual retailers," Emergent said in a statement. "Our pricing for both public interest groups and retailers would be significantly less than the current Wholesale Acquisition Cost."
SEE MORE: 3 in 10 adults say someone in family addicted to opioids, study finds
Making naloxone available more widely is seen as a key strategy to control the nationwide overdose crisis, which has been linked to more than 100,000 U.S. deaths a year. The majority of those deaths are tied to opioids, primarily potent synthetic versions such as fentanyl that can take multiple doses of naloxone to reverse.
Emergent said the drug will hopefully prevent some of the overdose deaths, but more work needs to be done to combat the ongoing opioid epidemic.
"OTC availability is one of several elements that can help to remove barriers to naloxone," the company said. "But the magnitude of the opioid epidemic requires additional support and pathways to access."
Trending stories at Scrippsnews.com